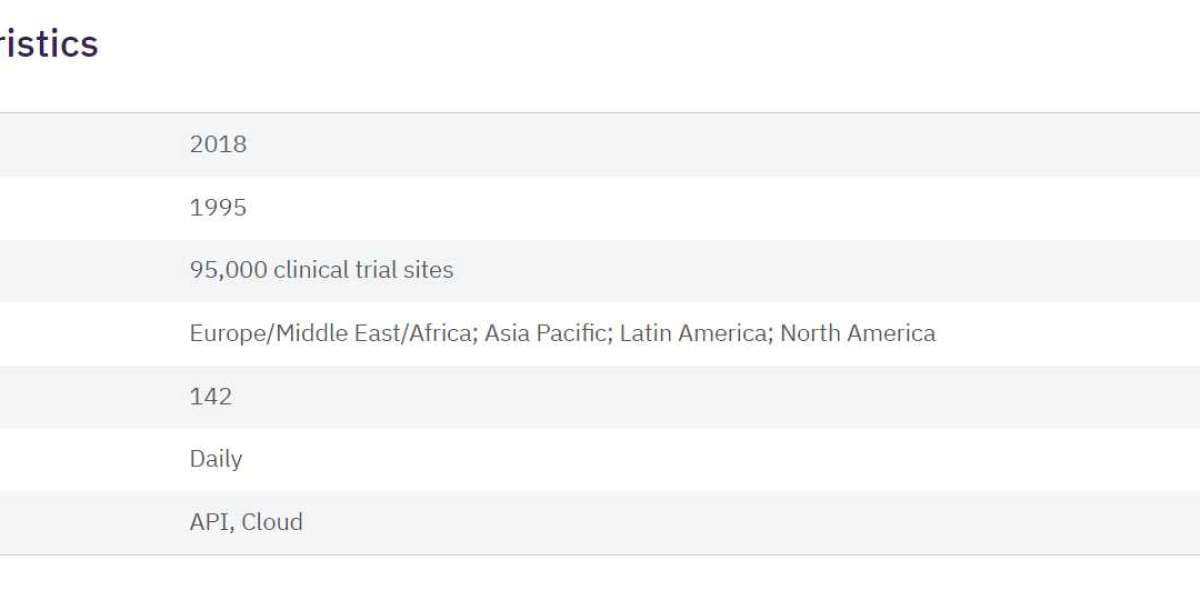
In the ever-evolving landscape of the pharmaceutical industry, the importance of efficient clinical trials cannot be overstated. Clinical trials play a crucial role in evaluating the safety and efficacy of new medications, and their success heavily relies on the selection of the right trial sites. With the advent of technology, pharmaceutical companies now have access to a powerful tool known as the Pharma Trial Sites Database. In this article, we will delve into the significance of this database, its impact on the clinical trial process, and the key factors to consider when choosing the best one.
1. Introduction: The Crucial Role of Clinical Trial Sites
Clinical trial sites serve as the foundation for conducting successful clinical trials. These sites are typically hospitals, research institutions, or medical centers where patients are enrolled, monitored, and treated during the trial process. The selection of appropriate trial sites is vital for accurate data collection, patient safety, and ensuring reliable trial results.
2. The Need for a Pharma Trial Sites Database
As the number of clinical trials increases and the global reach of pharmaceutical companies expands, the need for a centralized and reliable database becomes evident. A Pharma Trial Sites Database offers a comprehensive and up-to-date repository of potential trial sites, empowering pharmaceutical companies to make informed decisions and streamline the trial site selection process.
3. Advantages of a Comprehensive Database
Streamlined Site Selection Process
A Pharma Trial Sites Database simplifies the site selection process by providing detailed information about potential trial sites. This includes location, infrastructure, expertise, patient demographics, and previous trial experience. With this data at their fingertips, pharmaceutical companies can identify the most suitable trial sites based on their specific requirements, leading to more targeted and efficient site selection.
Improved Collaboration and Communication
Efficient communication and collaboration between various stakeholders are crucial for successful clinical trials. A comprehensive database enhances these interactions by offering contact details of trial site personnel, facilitating seamless communication between sponsors, investigators, and site staff. This real-time collaboration minimizes delays, promotes transparency, and ensures that trial activities run smoothly.
Enhanced Recruitment and Enrollment
Patient recruitment and enrollment are significant challenges in clinical trials. A Pharma Trial Sites Database provides valuable insights into trial sites with a large and diverse patient population. This enables pharmaceutical companies to identify sites with the necessary patient demographics, making it easier to meet recruitment targets within the specified timeline. Improved recruitment and enrollment accelerate the trial process and contribute to faster availability of new treatments.
Efficient Data Management
Data management is a critical aspect of clinical trials. A Pharma Trial Sites Database offers integrated data management capabilities, simplifying the collection, storage, and analysis of trial data. It ensures that data is securely stored and readily accessible to authorized personnel, minimizing errors and promoting data accuracy and integrity throughout the trial.
4. Key Features to Look for in a Pharma Trial Sites Database
When choosing a Pharma Trial Sites Database, several key features should be considered to ensure its effectiveness and suitability for your organization