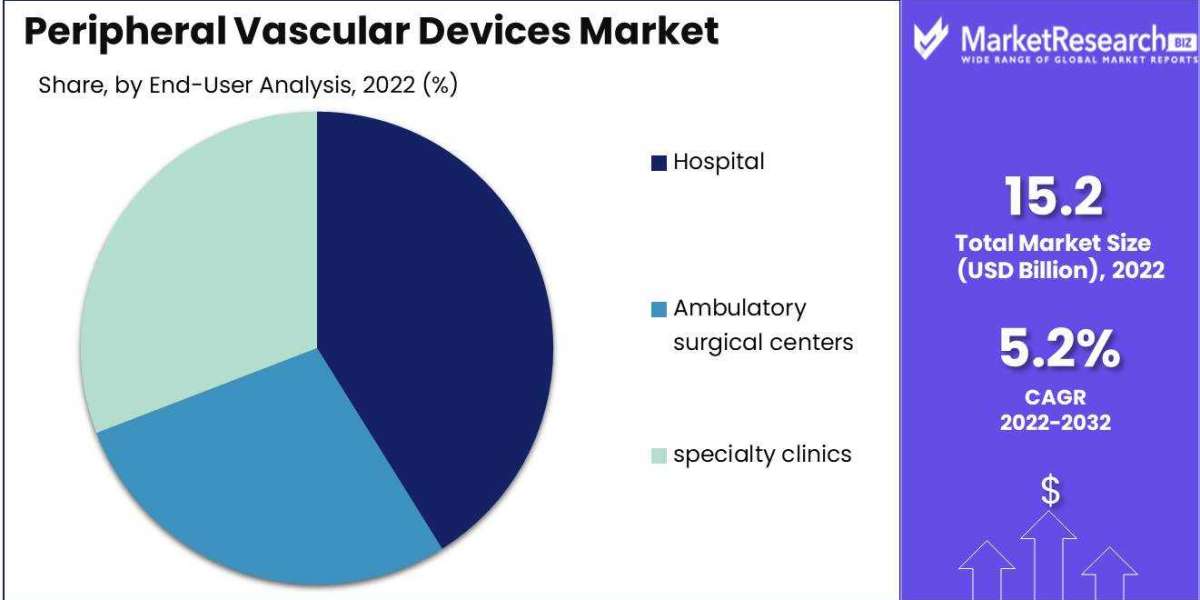
Peripheral Vascular Devices size is expected to be worth around USD 24.9 Bn by 2032 from USD 15.2 Bn in 2022, growing at a CAGR of 5.2% during the forecast period from 2023 to 2032.
Overview of Peripheral Vascular Devices:
Peripheral vascular devices are medical devices used to diagnose and treat disorders related to the circulatory system outside of the heart and brain, specifically disorders affecting extremities like legs and arms. Peripheral vascular diseases encompass an array of conditions like peripheral artery disease (PAD), deep vein thrombosis (DVT), and varicose veins; peripheral vascular devices play an integral part in managing these conditions by increasing blood flow, decreasing pain levels, and avoiding potential complications.
Key Market Segments
By Type Analysis
- Peripheral Stents
- PTA Balloons
- Catheters
- Peripheral Accessories
By End-User Analysis
- Hospital
- Ambulatory surgical centers
- specialty clinics
Rising Incidence of Vascular Diseases:
One of the primary drivers of growth in the peripheral vascular devices industry is an increasing prevalence of vascular diseases. Due to sedentary lifestyles, an aging population, and risk factors like obesity and diabetes, more people than ever before are becoming diagnosed with PAD, DVT, and varicose veins; creating an ever-increasing patient pool that creates substantial demand for peripheral vascular devices that can effectively diagnose and treat such ailments.
Top Key Players in the Peripheral Vascular Devices
- Abbott Laboratories
- Angioscore Inc.
- Edward Lifesciences Corporation
- Medtronic Inc.
- Jude Medical
- Teleflex Medical
- Volcano Corporation
- Boston Scientific Corporation
- Teleflex Medical
- Cook Group Inc.
- Cordis Corporation
- Covidien