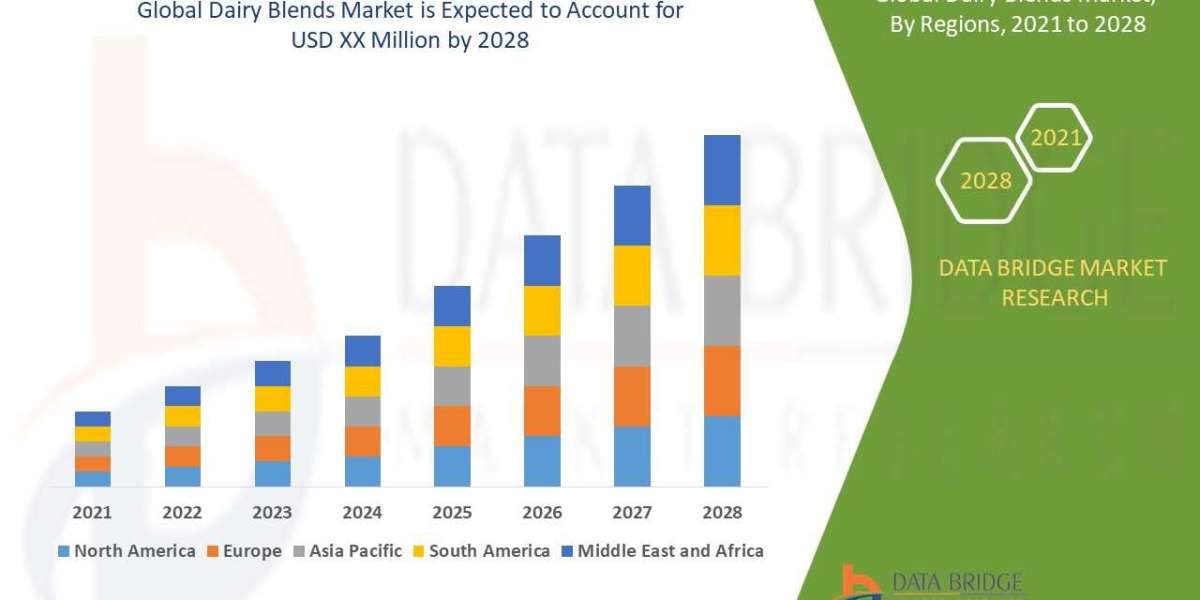
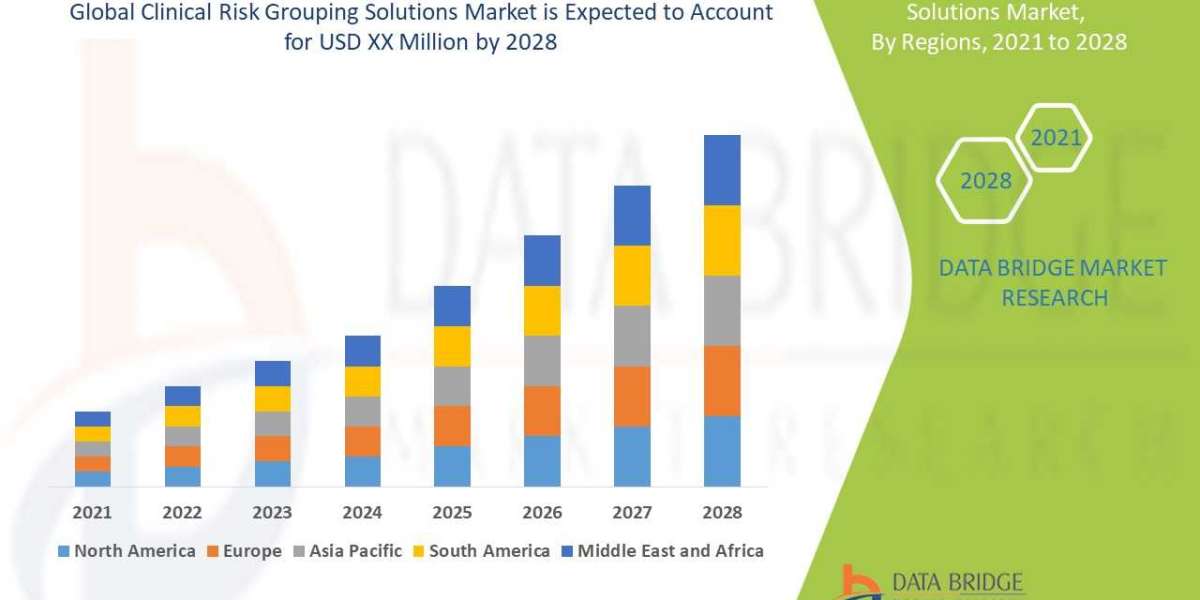
The pulmonary fibrosis biomarkers industry is projected to be valued US$ 3.9 billion in 2023. It is anticipated that the market will grow at a 4.2% CAGR to reach US$ 6.2 billion by 2033. Globally, the prevalence of pulmonary fibrosis is increasing, mostly as a result of ageing populations, occupational exposures, environmental pollutants, and genetic predispositions. Effective biomarkers are in high demand in order to support early disease detection and monitoring due to the increasing prevalence of the condition.
There is ongoing research and development in the identification and validation of biomarkers specific to pulmonary fibrosis. These biomarkers include genetic markers, circulating proteins, inflammatory markers, and imaging-based markers. The development of novel biomarkers is expected to enhance the accuracy and efficiency of diagnosis and prognosis, enabling personalized treatment approaches.
Unlock Peak Performance - Get Your Sample Now: https://www.futuremarketinsights.com/reports/sample/rep-gb-17617
The healthcare industry is increasingly adopting precision medicine approaches, which involve tailoring treatments based on an individual's unique characteristics and biomarker profiles. Biomarkers play a crucial role in guiding treatment decisions and assessing the response to therapies. This shift towards precision medicine is likely to drive the demand for pulmonary fibrosis biomarkers.
There is an active pipeline of novel drugs and therapies being developed for the treatment of pulmonary fibrosis. Biomarkers play a vital role in clinical trials by helping to identify appropriate patient populations, monitor treatment responses, and assess the safety and efficacy of experimental drugs. The demand for biomarkers is expected to grow alongside the expansion of clinical trials in pulmonary fibrosis.
The rise in healthcare expenditure globally, coupled with the growing focus on chronic disease management, is likely to support the adoption of advanced diagnostic tools and biomarker-based tests. Governments, insurance companies, and healthcare providers are recognizing the value of early detection and personalized treatment approaches, leading to increased investment in pulmonary fibrosis biomarkers.
Key Takeaways:
- The pulmonary fibrosis biomarkers industry in the United States is predicted to reach US$ 1.5 billion by 2033, increasing at a 6.5% CAGR.
- The pulmonary fibrosis biomarkers industry in the United Kingdom is estimated to reach a market value of US$ 208.1 million, expanding at a CAGR of 4.4% by 2033.
- During the forecast period, the pulmonary fibrosis biomarkers industry in China is expected to reach a market value of US$ 244.6 million, securing a 7.7% CAGR.
- The pulmonary fibrosis biomarkers industry in Japan is predicted to reach US$ 258.1 million by 2033, increasing at a 5.2% CAGR.
- South Korea's pulmonary fibrosis biomarkers industry is predicted to achieve a market value of US$ 152.6 million, rising at a 7.0% CAGR during the forecast period.
- With a CAGR of 4.0% from 2023 to 2033, the pulmonary function tests is expected to dominate the pulmonary fibrosis biomarkers industry.
- With a CAGR of 4.2% from 2023 to 2033, the specialty clinics is expected to dominate the pulmonary fibrosis biomarkers industry.
Key Players:
- J. Mitra Co. Pvt. Ltd.
- Thermo Fisher Scientific Inc.
- Elabscience Biotechnology Inc.
- CUSABIO TECHNOLOGY LLC
- Wuhan Fine Biotech Co., Ltd.
- Company ABclonal, Inc.
- Nanjing Vazyme Biotech Co.,Ltd.
- RayBiotech Life, Inc.
- Hipro Biotechnology Co.,Ltd.
- Vitrosens Biotechnology
- bioMérieux SA
- ELK Biotechnology CO.,Ltd.
- Immuno-Biological Laboratories, Inc.
- BISAF
- Roche Diagnostics
Key Developments:
- In November 2020, Roche and bioMérieux announced a collaboration to improve antibiotic stewardship by developing and providing PCT assays on Roche's cobas platforms.
- In February 2020, bioMérieux received FDA clearance for the Vidas BRAHMS PCT assay, which is an automated immunoassay platform for measuring PCT levels.
Segmentation Analysis of the Procalcitonin (PCT) Assay Market
By Sample Type:
- Serum
- Plasma
- Cell Culture Medium
- Other Biological Fluids
By Product:
- Test Kits
- Procalcitonin ELISA test kits
- Procalcitonin CLIA kits
- PCT Immunochromatography KITS
- PCT Rapid Test Kit
- Analyzer Instruments
- Consumables
By Purpose:
- Clinical Use
- Research Use Only
By End User:
- Hospital
- Specialty Clinics
- Academic and Research Institute
- Diagnostics Laboratories
By Region:
- North America
- Latin America
- Europe
- East Asia
- South Asia
- Oceania
- The Middle East Africa